1. Kossel and Lewis approach to chemical bonding is based on the inertness of the noble gases which have little or no tendency to combine with other atoms.
2. They proposed that noble gases are stable due to their completely filled outer electronic configuration.
3. Elements other than noble gases try to attain the completely filled outer electronic configuration by losing, gaining or sharing one or more electrons from their outer shell.
4. For e.g., sodium loses one electron to form Na ion and chlorine accepts that electron to give chloride ion, Cl- .These two ions are held together by electrostatic attractive forces, a bond known as an electrovalent bond.
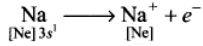
5. In diatomic molecules such as nitrogen and oxygen, they achieve the stable noble gas electronic configuration by mutual sharing of electrons.
6. Lewis introduced a scheme to represent the chemical bond and the electrons present in the outer shell of the atom called Lewis dot structure

7. For example, the electronic configuration of nitrogen is 1s2 2s2 2p3 . It has 5 electrons in its outer shell. The lewis structure of nitrogen is 
8. In N, molecule, equal sharing of 3 electrons from each nitrogen atom takes place as follows
