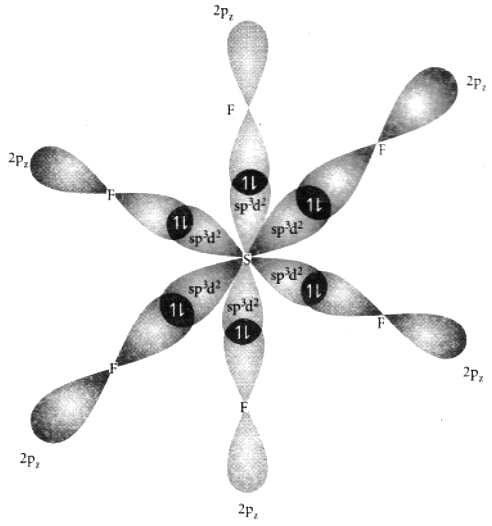
1. In sulphur hexafluoride SF6 , the central atom sulphur extend its octet to undergo sp3d hybridisation to generate six sp3d2 hybridised orbitals which accounts for six equivalent S – F bonds.
2. The ground state electronic configuration of sulphur is [Ne] 3s23px13py13pz1

3. One electron each form 3s orbital and 3p orbital of sulphur is promoted to its two vacant 3d orbitals dz2 and dx2-y2 in the excited state.

4. A total of six valence orbitals from sulphur (one 3s orbital, three 3p orbitals and two 3d orbitals) (dx2 and dx2-y2) which mixes to give six equivalent sp3d2 hybridised orbitals. The orbital geometry is octahedral.
5. The six sp3d2 hybridised orbitals of sulphur overlaps linearly with 2pz orbital of six fluorine atoms to form the six S – F bonds in sulphur hexa fluoride