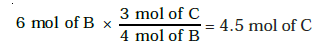2A + 4B → 3C + 4D
According to the above equation, 2 mols of ‘A’ require 4 mols of ‘B’ for the reaction.
Hence, for 5 mols of ‘A’, the moles of ‘B’ required

But we have only 6 mols of ‘B’, hence, ‘B’ is the limiting reagent. So amount of ‘C’ formed is determined by amount of ‘B’.
Since 4 mols of ‘B’ give 3 mols of ‘C’. Hence 6 mols of ‘B’ will give