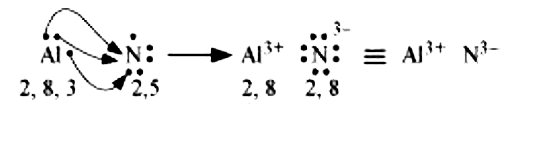(a) K and S:
The electronic configuration of K and S are as follows :
K:2,8,8,1
S:2,8,6
`K*" " :underset(..)overset(..)S:`
Sulphur (S) requires 2 more electrons to complete its octet. Potassium (K) requires one electrons more than the nearest noble gas i.e., Argon. Hence, the electron transfer can be shown as:

(b) Ca and O:
The electronic configuration of Ca and O are as follows:
Ca:2,8,8,2
O: 2,6
Oxygen requires two electrons more to complete its octet, whereas calcium has two electrons more than the nearest noble gas i.e., Argon. Hence, the electron transfer takes place as :

(c) Al and N:
The electronic configuration of Al and N are as follows:
Al:2,8,3
N:2,5
Nitrogen is three electrons short of the nearest noble gas (Neon), whereas aluminium has three electrons more than Neon. Hence, the electron transference can be shown as :