(1) The carbon atom adjacent to carbonyl carbon atom is called a-carbon atom (α – C) and the hydrogen atom attached to a-carbon atom is called α-hydrogen atom (α – H).

(2) The α-hydrogen of aldehydes and ketones is acidic in nature due to
(i) the strong-I effect of carbonyl group
(ii) resonance stabilization of the carbanion.

(3) Aldol condensation reaction is characteristic reaction of aldehydes and ketones containing active α-hydrogen atom.
(4) When aldehydes or ketones containing α – H atoms are warmed with a dilute base or dilute acid, two molecules of them undergo self condensation to give β-hydroxy aldehyde (aldol) or β-hydroxy ketone (ketol) respectively. The reaction is known as Aldol addition Reaction.
(5) In aldol condensation, the product is formed by the nucleophilic addition of αcarbon atom of a second molecule which gets attached to carbonyl carbon atom of the first molecule and α-hydrogen atom of the second molecule gets attached to carbonyl oxygen atom of the first molecule forming (- OH) group to give β-hydroxy aldehyde or ketone.
(6) This is a reversible reaction, establishing an equilibrium favouring aldol formation to a greater extent than ketol formation.
(7) For aldehyde :
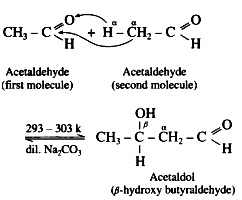
Acetaldol on heating undergoes subsequent elimination of water giving rise to α, β unsaturated aldehyde.

The overall reaction is called aldol condensation. It is a nucleophilic addition elimination reaction. For ketone :

Diacetone alcohol on dehydration by heating forms α, β unsaturated ketone.
