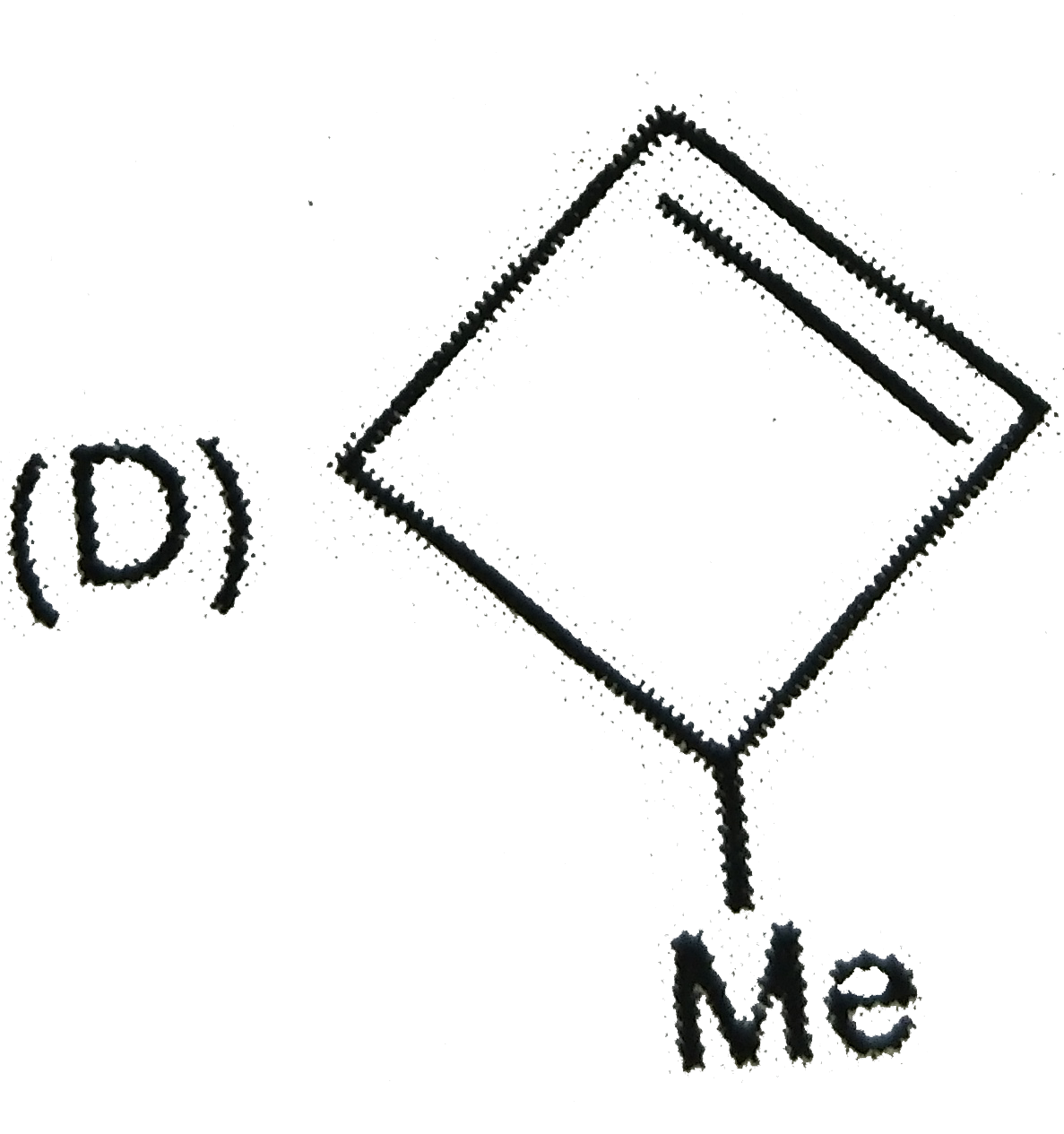The elimination reactions mainly involve three mechanism E1,E2 and E1cB. If the leaving group departs before `beta`-proton (`H^Theta` ion) then it is E1 mechanism. If proton is taken off first before leaving group it is E1cB mechanism.The pure E2 involves both `beta`-Hydrogen and leaving group departing simutaneously. If acidity of `beta`-Hydrogen increases and leaving group ability decreases then E1cB mechanism increases.

The alkene formed as a major product in the above elimination reaction is
A. `CH_2=CH_2`
B. `CH_3-CH_2-CH=CH_2`
C.

D.