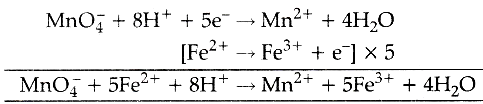Potassium permanganate is prepared from pyrolusite ore with KOH in the presence of oxidising agent like KNO3. The dark green potassium manganate undergoes electrolytic oxidation to produce potassium permanganate.

Oxidising nature of potassium permanganate can be shown as :
Ferrous to ferric