Correct option (A) Cl- ions are precipitated together
Explanation :
The solution contains 0.1 M each of Cl- and CrO42-
The concentration of Ag- required to ppt.

& the concentration of Ag+ required to ppt.

As the concentration of Ag- required to precipitate Cl- is less than that required to precipitate CrO42- , so Cl- ions starts precipitating first.
When the second ion starts precipitating the amount of Cl- left is
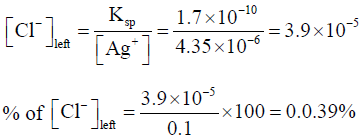
Thus % of [Cl] precipitated when CrO42- starts precipitating = 100 - 0.039 = 99.961%