The correct option is: (a) -93 kJ mol-1
Explanation:
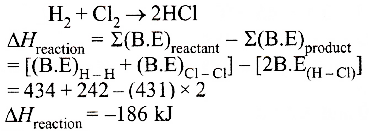
Heat of formation is the amount of heat absorbed or evolved when one mole of substance is directly obtained from its constituent element.
Hence, enthalpy of formation of HCI = -186/2 kJ
= -93 kJ mol-1