The correct option is (c) A is true but R is false.
Explanation:
Ferrocyanide ion
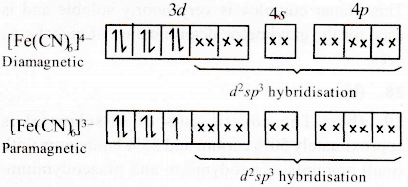
In ferrocyanide ion, the oxidation state of Fe is +2 however in ferricyanide ion, the oxidation number of Fe is +3.
Generally, the higher the oxidation state of the metal, the greater the crystal field splitting. It means crystal field splitting in ferrocyanide ion is lower than that of ferricyanide ion.