Temperature of saturation steam = 200 + 273 = 473 K
Initial temperature of gases = 1000 + 273 = 1273 K
Final temperature of gases = 500 + 273 = 773 K
For gases : cpg = 1 kJ/kg K
Latent heat of steam of 200°C
saturation temperature, hfg = 1940.7 kJ/kg
Atmospheric temperature = 20 + 273 = 293 K
Heat lost by gases = Heat gained by 1 kg saturated water when it is converted to steam at 200°C.
mgcpg (1273 – 773) = 1940.7
[where mg = mass of gases, cpg = specific heat of gas at constant pressure]
mg = \(\cfrac{1940.7}{(1273-773)}\) = 3.88 kg
Change of entropy of mg kg of gas,

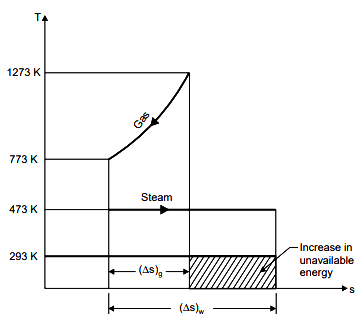
Change of entropy of water (per kg) when it is converted into steam,

Net change in entropy due to heat transfer
= – 1.935 + 4.103 = 2.168 kJ/K.
Increase in unavailable energy due to heat transfer
= 293 × 2.168, i.e., cross hatched area
= 635.22 kJ per kg of steam formed.