Correct option is (A) 1 and 3

The intermediate (1) is more stable then intermediate (2), because +R effect is more powerful then +H effect. and also in case of (1) we got a structure which have complete octet but in case of (2) we do not get such structure.
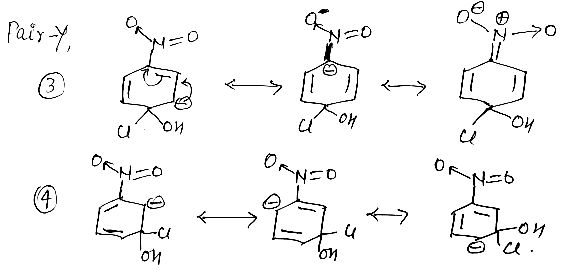
intermediate (3) is more stable than intermediate (4) because, in intermediate (3) the negative charge in conjugation with NO2 group, which stabilize intermediate (3) but in case (4) negative charge not in conjugation with -NO2 group.