The following diagram shows an experiment to study the action of dilute sulphuric acid on zinc granules.
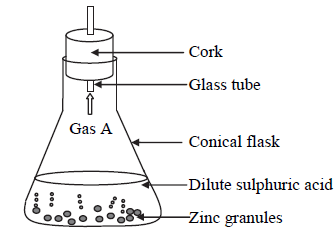
i. Identify the gas A.
ii. Write the chemical equation of the reaction that takes place and identify the type of reaction.
iii. What will happen if dilute hydrochloric acid is used instead of dilute sulphuric acid?