The graph shows the variation of temperature (T) of one kilogram of a material with the heat (H) supplied to it, At O, the substance is in the solid state. From the graph, we can conclude that...
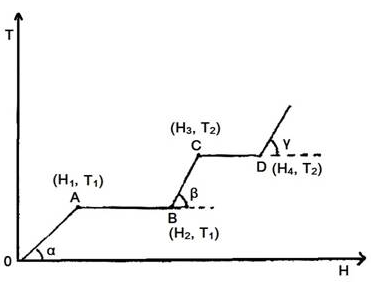
(A) T2 is the melting point of the solid.
(B) BC represents the change of state from solid to liquid.
(C) (H2 – H1) represents the latent heat of fusion of the substance.
(D) (H3 – H1) represents the latent heat of vaporization of the liquid.