(i) Average rate of reaction between interval of time 30 to 60 second is given by

Minus sign shows that rate of reaction is decreasing with time as conc. of ester is decreasing with time.
(ii) Pseudo first order rate constant k is given by
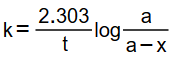
where a is initial conc. and (a – x) conc. after time t. Here a = 0.55 M.

The nearly equal values of k confirms that reaction is of first order. The actual value of rate constant is the average of three values of k. Therefore, rate constant of reaction
