The number of moles of a solute dissolved in 1 L (1000 ml) of the Solution is known as the molarity of the Solution.
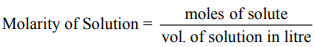
Molarity is a unit that depends upon temperature. It varies inversely with temperature.
Mathematically: Molarity decreases as temperature increases.

• If a particular Solution having volume V1 and molarity M1 is diluted to V2 mL and since number of moles of solute will remain constant, so, M1V1 = M2V2 M2: Resultant molarity
• If a Solution having volume V1 and molarity M1 is mixed with another Solution of same solute having volume V2 mL & molarity M2
