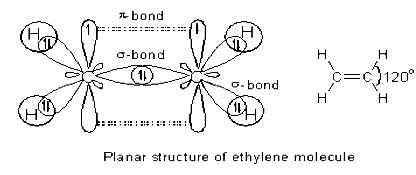
During the formation of ethylene molecule, each carbon atom undergoes sp2 hybridization in its excited state by mixing 2s and two 2p orbitals to give three half filled sp2 hybrid orbitals oriented in trigonal planar symmetry.
There is also one half filled unhybridized 2pz orbital on each carbon perpendicular to the plane of sp2 hybrid orbitals.

The carbon atoms form a σsp2-sp2 bond with each other by using sp2 hybrid orbitals. A πp-p bond is also formed between them due to lateral overlapping of unhybridized 2pz orbitals. Thus there is a double bond (σsp2-sp2 & πp-p) between two carbon atoms. Each carbon atom also forms two σsp2-s bonds with two hydrogen atoms. Thus ethylene molecule is planar with ∠HCH & ∠HCC bond angles equal to 120°.
All the atoms are present in one plane.