A container fitted with frictionless massless piston consist of five valves-I, II, III, IV and V These valves open automatically if pressure exceed over 1.5, 2.2, 2.5, 4.4 and 4.8 atm respectively. Under the given initial conditions (mentioned in given diagram) system is in state of equilibrium Piston is now pressed in downward direction very slowly.
[Note: Consider the diameter of value tube negligible and temperature remain constant.]
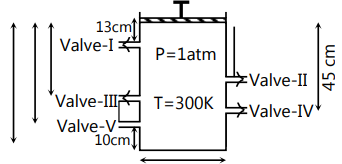
Select the correct option(s).
(A) Value-II will be opened first
(B) As the piston crosses the valve which will be opened first, the remaining number of moles in container are 5/3.
(C) Valve-V will be the second valve which is open
(D) Number of moles will zero as piston crosses Valve-V