An ideal monatomic gas is at P0 , V0 . It is taken to final volume 2V0 when pressure is P0 /2 in a process which is straight line on P-V diagram. Then
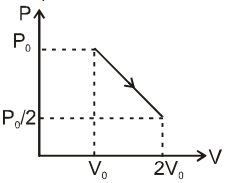
(A) The final temperature is equal to initial temperature
(B) There is no change in internal energy
(C) The straight line will be tangent on isothermal curve at V = 3V0 / 2
(D) The straight line will be tangent on adiabatic curve at V = 10V0 / 3