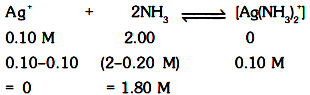
It is assumed that all Ag+ ions have been complex and only x amount is left

x = 1.93 × 10–9 M = [Ag+] undisolved
[Cl–] = 1.0 × 10–2 M
∴ [Ag+] [Cl–] = 1.93 × 10–9 × 1.0 × 10–2 = 1.93 × 10–11 < 1.8 × 10–10 [Ksp(AgCl)]
Hence, AgCl (s) will not precipitate.