What is Borax?
Borax, also termed as tincal, sodium tetraborate decahydrate (Na2B4O7·10H2O). A naturally-occurring mineral found in soil, plants, and even our own bodies. Borax is soft and lightweight, colourless crystalline substance, it is utilized in several ways—as a part of glass and pottery glazes within the ceramics business, as a solvent for metal-oxide slags in metallurgy, as a flux in welding and soldering, and as a fertilizer additive, a soap supplement, a disinfectant, a mouthwash, and a softener.
Borax is a salt of boric acid but it is not chemically the same as boric acid.
IUPAC name - Sodium tetraborate decahydrate
Is Borax Toxic?
There are a lot of contradictory factors grounded on the source.
Actual warnings relate to avoiding eye contact, undiluted skin contact and bodily function.
Borax is prohibited for food use by the FDA and also the ECA (European Chemicals Agency) thought of a substance of high concern however didn’t give any documentation aside from soil level dangers.
Borax is an efficient natural cleaner and a safer different to several standard cleaners. Yes, it's conjointly a pesticide, however a natural one (and nice at obtaining eliminate ants- here’s a good tutorial) however I’m however to search out conclusive proof that it's either safe or harmful to humans (other than if it's eaten, rubbed within the eyes, etc.).
Is Borax Safe to Use?
Borax may be a natural ingredient, however as we've already distinguished, it's still hepatotoxic, if eaten. Meaning you ought to keep it out of reach of kids and pets. If you utilize it to form your own product, make sure to label the containers with all the ingredients, simply just in case there is ever an accidental intake. You’ll grasp what is in your concoction, however people in your home might not.
Treat borax with identical care that you simply would the other cleansing product, and you should not have any issues.
Borax within the usual variety of sodium tetraborate decahydrate isn't acutely unhealthful, which suggests an outsized quantity would wish to be indrawn or eaten to provide health effects. As far as pesticides go, it's one in all the protection chemicals accessible. A 2006 analysis of the chemical by the U.S. EPA found no signs of toxicity from exposure and no proof of toxicity in humans. Not like several salts, skin exposure to borax doesn't turn out skin irritation.
However, this does not create borax unconditionally safe. The foremost common downside with exposure is that inhaling the dust will cause metabolic process irritation, significantly in kids. Ingesting large amounts of borax will cause nausea, vomiting, and diarrhoea. The European Union (EU), Canada, and Indonesia contemplate borax and boric acid exposure a possible health risk, primarily because people are exposed to it from many sources in the diet and from surroundings. The priority is that overexposure to a chemical usually deemed safe might increase risk of cancer and harm fertility.
While the findings are somewhat contradictory, it's recommended kids and pregnant girls limit their exposure to borax if doable.
Preparation of Borax:
1) From tincal
Naturally occurring borax is termed as tincal or suhaga. Tincal obtained from dried up lakes contains 50% borax. It is boiled with water and filtered to get rid of insoluble impurities of clay ,sand. The filtrate is focused when crystals of borax dispersed out.
2) From Colemanite
The crystal colemanite is finely crushed and is heated with sodium carbonate solution.
Ca2B6O11 + 2 Na2CO3 ——–> Na2B4O7 + 2 NaBO2 + 2 CaCO3 ↓
The precipitate of calcium carbonate thus formed is detached by filtration. The filtrate is focused and chilled when crystals of borax isolated out. Sodium metaborate present in the mother liquor can be converted into borax by passing a current of carbon dioxide through it.
3) From boric acid
Borax can also be prepared in small amounts by neutralising boric acid with sodium carbonate.
On cooling crystal of borax i.e. Na2B4O7.10H2O separate out.
Properties of Borax:
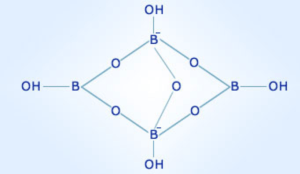
Chemical formula - Na2B4O7·10H2O or Na2[B4O5(OH)4]·10H2O
Molar mass - 381.38 (decahydrate) , 201.22 (anhydrate)
Appearance - white solid
Density - 1.73 g/cm3 (solid)
Melting point - 743 °C (1,369 °F; 1,016 K) anhydrate
Boiling point - 1,575 °C (2,867 °F; 1,848 K)
Crystal structure - Monoclinic Prismatic
1) It is a white crystalline solid, less soluble in cold water but more soluble in hot water.
2) The aqueous solution of borax is alkaline due to hydrolysis. Borax is therefore, used as a water softener and cleaning agent.
Na2B4O7 + 2 H2O ———-> 2 NaOH + H2B4O7
H2B4O7 + 5 H2O ———-> 4 H3BO3
3) Action of heat- borax bead test
Borax loses its water of crystallisation and swells up to form a puffy mass. On further heating, it melts into a clear liquid which solidifies to a transparent glass like bead which consists of sodium metaborate (NaBO2) and boric anhydride(B2O3)
Na2B4O7.10H2O ————–> Na2B4O7 + 10 H2O
Na2B4O7 ————-> 2 NaBO2 + B2O3
The glassy bead is commonly known as borax bead and is employed in qualitative analysis for the detection of certain coloured basic radicals such as Ni2+ , Co2+, Cr3+, Cu2+, Mn2+ . Whenever a coloured salt containing these cations is heated with borax bead on a platinum wire, the salt decomposes to form the corresponding metal oxide which then combines with B2O3 present in the glassy bead to form coloured metaborates. This test is called borax bead test.
CoSO4 ———> CoO + SO3
CoO + B2O3 ——> Co(BO2)2
NiO + B2O3 ———-> Ni(BO2)2
Cr2O3 + B2O3 ———-> 2 Cr(BO2)3
MnO + B2O3 ———-> Mn(BO2)2
CuO + B2O3 ———-> Cu(BO2)2
Action of sodium hydroxide
On adding a calculated quantity of sodium hydroxide to borax , sodium metaborate is formed.
Na2B4O7 + 2 NaOH ———–> Na2BO2 + H2O
Action of sulphuric acid
On adding a calculated quantity of concentrated sulphuric acid to a hot concentrated solution of borax, boric acid is produced.
Na2B4O7 + H2SO4 ———> Na2SO4 + H2B4O7
H2B4O7 + 5 H2O ——-> 4 H3BO3
Action of ethyl alcohol and Sulphuric acid
On heating borax with ethyl alcohol and concentrated sulphuric acid, vapours of triethylborates are produced. When ignited these vapours burn with a green edged flame.
Na2B4O7 + H2SO4 +5 H2O ———-> Na2SO4 + 4 H2BO3
H3BO3 + 3 C2H5OH ——-> B(OC2H5)3 + 3 H2O