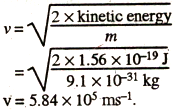Wavelength of the photon, λ = 4 x 10-7 m
Work function of the metal, W = 2.13 eV = 2.13 x 1.6020 x 10-19 J
= 3.14 x 10-19 J
(i) Energy of photon = ch/λ = {3 x 108 ms-1 x 6.626 x 10-34 Js}/{4 x 10-7 m}
= 4.97 x 10-19 J
= {4.97 x 10-19 J}/{1.6020 x 10-19 J/eV} = 3.10 eV
(ii) Kinetic energy of the emission = Ephoton - W
= 4.97 x 10-19 J - 3.41 x 10-19 J
= 1.56 x 10-19 J
= 3.10 eV - 2.13 eV = 0.97 eV
(iii) Kinetic energy of the emitted photoelectron = 1/2 m x v2
or,