Let the initial pressure, pi = p1
initial volume, Vi = V1
final volume, Vf = V2
= 2V1
Work done in isothermal process,
Wiso = 2.303 RT log \((\frac{v_2}{v_1})\)
Since PV = RT (1 mol of gas),
Wiso = P1 V1 ln2 ......(1)
work done in adiabatic process,

For adiabatic process,
P1 \({V_1^γ}\) =P1 \(V_2^γ\) K (constant)

Since work done is same for both processes, Wiso = Wad
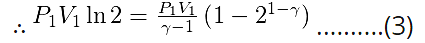
Re-arranging and simplifying (3),
21-γ - 1 = (1 - γ) ln2.