(a) Oxidising and Reducing Nature : Hydrogen peroxide acts as a strong oxidising agent in both acidic and basic medium. In acidic medium, oxidation is slow while in basic medium, it is fast.
e.g., PbS(s) + 4H2O2 (aq) ➝ PbSO4 (s) + 4H2O(l) [in acidic medium]
MnSO4 + H2O2 + 2NaOH ➝ MnO2 + Na2SO4 + 2H2O [in basic medium] Hydrogen peroxide acts as a strong reducing agent for strong oxidising substances in both acidic and basic medium. The rate of reaction is slower in acidic medium as compared to basic medium.
e.g., K2Cr2O7 + 4H2SO4 + 3H2O2 ➝ K2SO4 + Cr2(SO4)3 + 7H2O + 3O2 [in acidic medium]
I2 + H2O2 + 2OH– ➝ 2I– + 2H2O + O2 [in basic medium]
(b) Addition Reactions : Ethene reacts with hydrogen peroxide to form an addition product i. e., ethylene glycol.

(c) Formation of Peroxide : Dehydrated hydrogen peroxide (acidic in nature) reacts with base to form salts (peroxide).
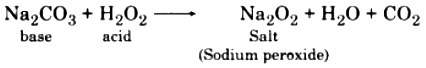
(d) Decomposition: Pure hydrogen peroxide is unstable. So, when it is placed in open air or on heating, it dissociates into H2O and oxygen.
2H2O2 ➝ 2H2O + O2
It is an exothermic reaction, which can be catalysed by Pt, Co, Au, Cu etc. It can be controlled by addition of small quantity of acid, alcohol or acetanilide.