In beta decay, a radioactive nucleus emits either electron or positron. If electron (e-) is emitted, it is called β- decay and if positron (e+) is emitted, it is called p+ decay. The positron is an anti-particle of an electron whose mass is same as that of electron and charge is opposite to that of electron – that is, +e. Both positron and electron are referred to as beta particles.
1. β decay:
In β decay, the atomic number of the nucleus increases by one but mass number remains the same. This decay is represented by

It implies that the element X becomes Y by giving out an electron and antineutrino (\(\bar v\)). In otherwords, in each β- decay, one neutron in the nucleus of X is converted into a proton by emitting an electron (e-) and antineutrino. It is given by
n → p + e- +\(\bar v\)
Where p -proton, \(\bar v\) -antineutrino.
Example: Carbon (\(^{14}_6C\) ) is converted into nitrogen (\(^{14}_7N\) ) through β- decay

2. β+ decay:
In p+ decay, the atomic number is decreased by one and the mass number remains the same. This decay is represented by
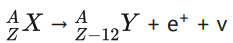
It implies that the element X becomes Y by giving out an positron and neutrino (v). In otherwords, for each β+ decay, a proton in the nucleus of X is converted into a neutron by emitting a positron (e+) and a neutrino. It is given by
p → n + e+ + v
However a single proton (not inside any nucleus) cannot have β+ decay due to energy conservation, because neutron mass is larger than proton mass. But a single neutron (not inside any nucleus) can have β- decay.
Example: Sodium (\(^{23}_{11}Na\) ) is converted into neon (\(^{22}_{10}Ne\)) decay.
