Potassium chloride (KCl) is the salt of a strong acid (HCl) and strong base (KOH). Hence, it is neutral in nature and does not undergo hydrolysis in normal water. It dissociates into ions as follows:

In acidified and alkaline water, the ions do not react and remain as such. Aluminium (III) chloride is the salt of a strong acid (HCl) and weak base [Al(OH)3]. Hence, it undergoes hydrolysis in normal water.
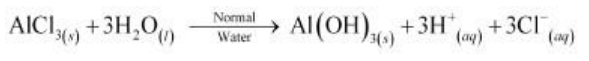
In acidified water, H+ ions react with Al(OH)3 forming water and giving Al3+ ions. Hence,

In alkaline water, the following reaction takes place:
