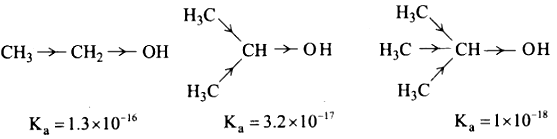
1. The acidic nature of the alcohol is due to the polar nature of O – H bond. When an electron withdrawing - I groups such as – Cl, – F etc… is attached to the carbon bearing the OH group, it withdraws the electron density towards itself and thereby facilitating the proton donation.
2. In contrast, the electron releasing group such as alkyl group increases the electron density on oxygen and decreases the polar nature O – H bond, I lence it results in the decrease in acidity.
3. On moving from primary to secondary and tertiary alcohols, the number of alkyl groups which attached to the carbon bearing -OH group increases, which results in the following order of acidity.
1°alcohol > 2° alcohol > 3° >alcohol
For example