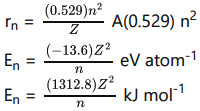Assumptions of Bohr atom model.
i. The energies of electrons are quarantined
ii. The electron is revolving around the nucleus in a certain fixed circular path called stationary orbit.
iii. Electron can revolve only in those orbits in which the angular momentum (mvr) of the electron must be equal to an integral multiple of h/2π
mvr = nh/2π, where n = 1,2,3,…etc.,
iv. As long as an electron revolves in a fixed stationary orbit, it doesn’t lose its energy. But if an electron jumps from higher energy state (E2 ) to a lower energy state (E1 ), the excess energy is emitted as radiation. The frequency of the emitted radiation is E2 – E1 = hv.
∴ v = (E2 – E1) / h
Conversely, when suitable energy is supplied to an electron, it will jump from lower energy orbit to a higher energy orbit.
v. Bohr’s postulates are applied to a hydrogen like atom (H, He+ and Li2+ etc..) the radius of the nth orbit and the energy of the electron revolving in the th orbit were derived.