The relation of two or more compounds, radicals, or ions that are composed of the same kinds and numbers of atoms but differ from each other in structural arrangement (structural isomerism) as CH3 OCH3 and CH3 CH2 OH, or in the arrangement of their atoms in space and therefore in one or more properties.

(a) Geometric isomerism:
This type of isomerism is common in heteroleptic complexes. It arises due to the different possible geometric arrangements of the ligands. For example:
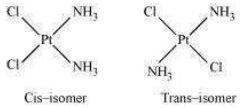
(b) Optical isomerism:
This type of isomerism arises in chiral molecules. Isomers are mirror images of each other and are non-superimposable.

(c) Linkage isomerism: This type of isomerism is found in complexes that contain
ambidentate ligands. For example:
[Co(NH3)5 (NO2)]Cl2 and [Co(NH3)5 (ONO)Cl2
Yellow form Red form (d)
Coordination isomerism:
This type of isomerism arises when the ligands are interchanged between cationic and anionic entities of differnet metal ions present in the complex.
[Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] (e)
Ionization isomerism:
This type of isomerism arises when a counter ion replaces a ligand within thecoordination
sphere. Thus, complexes that have the same composition, but furnish different ions when
dissolved in water are called ionization isomers. For e.g.,
Co(NH3)5SO4)Br and Co(NH3)5Br]SO4.
(f) Solvate isomerism:
Solvate isomers differ by whether or not the solvent molecule is directly bonded to the metal ion or merely present as a free solvent molecule in the crystal lattice.
[Cr[H2O)6]Cl3 [Cr(H2O)5Cl]Cl2 H2O [Cr(H2O)5Cl2]Cl2H2O
Violet Blue-green Dark green