Structure of mononuclear metal carbonyls:
The mononuclear metal carbonyls have only M-C≡O linear linkage and their structure depends upon the coordination number of metal, i.e., number of carbonyl groups attached to the metal and the type of hybridization on central metal. The structure of Ni (CO) 4 has been established to be tetrahedral from various studies. The nickel atom of this complex is sp3 hybridised.
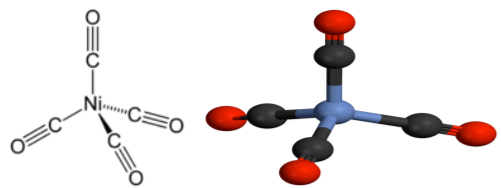
[Co(CO)4] - and [Fe(CO)4] 2- are isoelectronic to [Ni(CO)4] and hence they also have tetrahedral structure.
The pentacarbonyls such as Fe(CO)5 , Ru(CO)5 and Os(CO)5 have trigonal bipyramidal structure arising from dsp3 hybridisation on the central metal.

[Mn(CO)5] - is isoelectronic to Fe(CO)5 and it also has trigonal bipyramidal structure. The hexacacarbonyls such as V(CO)6 , Cr(CO)6 , Mo(CO)6 and W(CO)6 have octahedral structure arising from d2 sp3 hybridisation on the central metal

[V(CO)6] - , [Nb(CO)6] - , [Ta(CO)6] - and [Mn(CO)6] + also have octahedral structure .