1. (i) Ethane – 7σ bonds
(ii) Acetylene-3σ bonds and 2 π bonds
2. In BF molecule, the ground state electronic configuration of central boron atom is 1s²2s²2p¹. In the excited state, one of the 2s electrons is promoted to vacant 2p orbital. As a result, boron has three unpaired electrons. These three orbitals (one 2s and two 2p) hybridise to form three sp2 hybrid orbitals oriented in a trigonal planar arrangement and overlap with 2 p orbitals of F to form three B – F bonds. Therefore, BF3 molecule has a planar geometry with FBF bond angle of 120°.

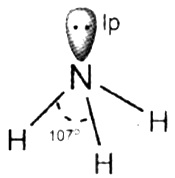
In NH3 , the valence shell electronic configuration of N in ground state is 2s²2p\(\frac{1}{x}\) 2p\(\frac{1}{y}\) 2p\(\frac{1}{z}\) . These four orbitals undergo sp³ hybridisation to form four sp³ hybrid orbitals, three of them containing unpaired electrons and the fourth one containing lone pair. The three hybrid orbitals overlap with 1 s orbitals of hydrogen atoms to form three N – H sigma bonds. Since, the bp-lp repulsion is greater than the bp-bp repulsion, the molecule gets distorted and the bond angle is reduced to 107° from 109.5°. Thus, the geometry of NH3 molecule is trigonal pyramidal.