Answer is (3) 0
In presence of SFL △0 > p means pairing occurs therefore
For Fe2+ → 3d6
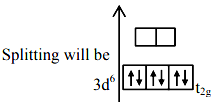
∴ No of unpaired e-(s) = 0
∴ μ = \(\sqrt{n(n+2)}BM = 0\)
[n = No of unpaired e– (s)]
In NiCl2 Ni+2 is having configuration 3d8
∴ Number of unpaired electron = 2
After formation of oxidised product
[Ni(CN)6] –2 Ni+4 is obtained
Ni+4 ⇒ 3d6 and CN– is strong field ligand
∴ number of unpaired electrons = 0
∴ The charge is 2 – 0 = 2