Correct option: (c) [Ni(H2O)6]2+
The electronic configurations of Ni2+ is 3d8 .

Electronic configuration of Cr2+ is 3d4 .

Electronic configuration of Mn2+ is 3d5 .

Electronic configuration of Fe2+ is 3d6 .
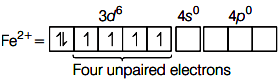
Number of unpaired electrons is minimum in [Ni(H2O)6]2+, thus it show minimum paramagnetic behaviour.