i. Crystalline solids:
a. A crystalline solid usually consists of a large number of small crystals, each of them having a definite characteristic geometrical shape. These tiny crystals are called unit cells.
b. A unit cell is a basic repeating structural unit of a crystalline solid.
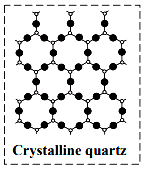
c. Crystalline solids have long range order which means that there is a regular pattern of arrangement of particles (atoms, ions or molecules) which repeats itself periodically over the entire crystal.
eg. Sodium chloride and quartz
ii. Amorphous solids:
a. The arrangement of constituent particles (atoms, molecules or ions) in amorphous solids has only short range order.
b. In such an arrangement, a regular and periodically repeating pattern is observed over short distances only.
c. Such portions are scattered and in between, arrangement is disordered.
eg. glass, rubber, plastics and amorphous silicon.
