Preparation of KMnO4 from pyrolusite ore (MnO2):
Step I: Conversion of pyrolusite ore (MnO2) into potassium manganate.
The finely powdered pyrolusite mineral (MnO2) is fused with potassium hydroxide or potassium carbonate in the presence of air or oxidising agent such as potassium nitrate or potassium chlorate giving green coloured potassium manganate.
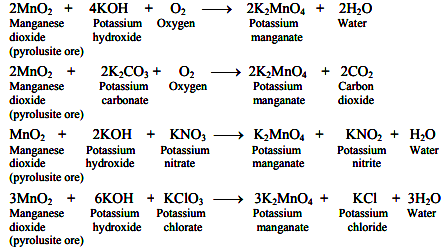
The reaction mixture containing K2MnO4 (potassium manganate) is treated with water and then converted into KMnO4 (potassium permanganate) either by oxidation or by electrolysis.
Step II: Oxidation of potassium manganate (K2MnO4) to potassium permanganate (KMnO4).
There are two methods for oxidation of potassium manganate.
i. Chemical oxidation: This can be achieved by any one of the following:

Note: The carbon dioxide process is uneconomical as one third of the original manganate is reconverted to manganese dioxide. However, this process has the advantage that the potassium carbonate formed as a byproduct can be used for the oxidative fusion of manganese dioxide. In the chlorine process, potassium chloride obtained as a byproduct is lost.
ii. Electrolytic oxidation: For manufacturing potassium permanganate commercially, the method of electrolytic oxidation is preferred. The alkaline manganate solution obtained in step (I) is electrolysed between iron electrodes separated by diaphragm.
The reactions taking place are as follows:

Thus, manganate ions are oxidized to permanganate at the anode and hydrogen gas is liberated at the cathode.

After the oxidation is complete, the solution is filtered and evaporated under controlled conditions to obtain the deep purple black crystals of potassium permanganate.