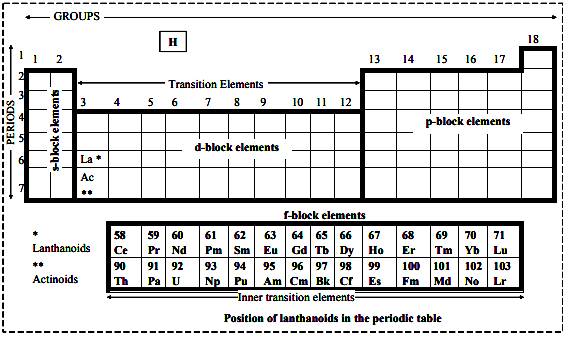
Position of lanthanoids in the periodic table:
i. The 14 elements (from 58 to 71) of lanthanoid series have been placed along with lanthanum (at no. 57) in the third column and sixth period of the periodic table.
ii. In the periodic table, as we move from one element to other, either from left to right or from top to bottom, the properties exhibit a gradual change. But these fifteen are so similar to one another, that they cannot be placed one after the other or one below the other.
iii. As the fourteen elements i.e., Ce(58) to Lu(71) are closely similar to La(57), the best place for them is along with Lanthanum (La) i.e., third column and sixth period in the periodic table.
iv. In case, these elements are given different position in order of their increasing atomic numbers, the symmetry of the periodic table would be disrupted.
v. Due to this reason, the lanthanoids are placed at the bottom of the periodic table with a reference to the third group in the sixth period.
vi. This position in the periodic table is justified due to following facts:
a. The number of valency electrons is same for all elements, i.e., one in 5d and two in 6s.
b. Group valency of all lanthanoids is 3.
c. Physical and chemical properties of all these elements are similar.
d. Atomic numbers of lanthanoids are in between lanthanum (57) and hafnium (72).