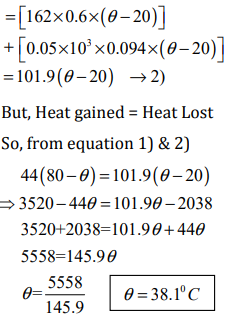Let θ0C = final temperature of the mixture.
Mass of ethyl alcohol = volume ×Density
= 200 × 0.81
Heat lost by Aluminum block = Mass X specific heat X fall in temperature

Heat gained by the ethyl alcohol and calorimeter = (Mass of ethyl alcohol × specific heat × change in Temperature) + Mass of copper calorimeter × specific heat X change in Temperature