(A)
(i) Sodium carbonate is a salt of weak acid and strong base.
(ii) In aqueous solution it undergoes hydrolysis.
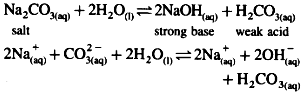
(iii) Strong base dissociates completely while weak acid dissociates partially since [OH-] > [H3O+], the solution is basic.
(B)
(i) Ammonium chloride is a salt of strong acid and weak base.
(ii) In aqueous solution it undergoes hydrolysis

(iii) Since [H+] or [H3O+] > [OH-] the solution is acidic.