Most of the chemical reactions and industrial processes are carried out in aqueous solutions, hence there is a need to know concentration of H+ and OH- ions in the solution.
Sorensen developed a convenient scale to represent the acidic, basic or neutral nature of the solution. The pH scale is used to express the concentration of H+ and OH- along with pH and pOH of the solution.
According to Sorensen,
pH = -log10[H+], pOH = -log [OH-]
pH + pOH = 14.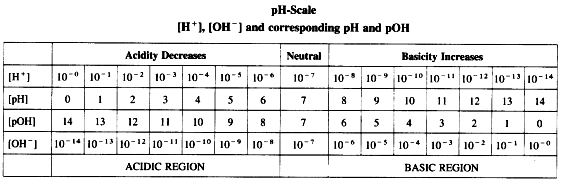
Acids, basic and neutral solutions.