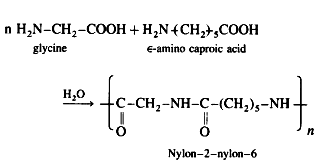
(1) It is a copolymer and has polyamide linkages. The monomers glycine and e-amino caproic acid undergo condensation polymerisation to form nylon-2-nylon-6.
Nylon-2-nylon-6 is used in orthopaedic devices and implants.
(2) Monomer glycine contains two carbon atoms and e amino caproic acid contains six carbon atoms, hence the polymer is termed as nylon-2-nylon-6.