Given,
\(ΔH^0_{O-H}\) = 464 kJ mol-1;
\(ΔH^0_{C-O}\) = 351 kJ mol-1.
The given reaction can be represented as,
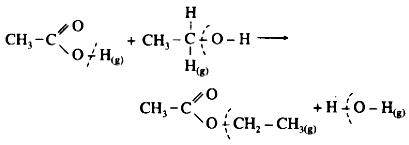
In this reaction 1O-H and 1C-0 bond of the reactants are broken while 1C-0 and 1O-H bonds of the products are formed.
Enthalpy of reaction,

Hence,
Enthalpy change for the reaction = ΔrH0 = 0.