When two or more reactants combine in a reaction to form a single product, it is called a combination reaction.
Examples:
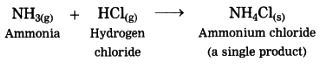
1. The ammonia gas reacts with hydrogen chloride gas to form the salt in gaseous state, immediately it condenses at room temperature and gets transformed into the solid state.
2. Magnesium burns in air to form white powder of magnesium oxide as a single product.
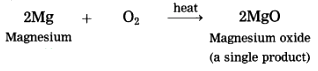
3. Iron reacts with sulphur to form iron sulphide.
