When oxidation and reduction take place simultaneously in a given chemical reaction, it is known as a redox reaction.
(1)

H2S is oxidised and SO2 is reduced.
(2)

CuO is reduced and H2 is oxidised.
(3)
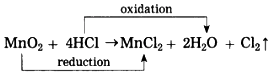
(1) Oxidation: H2S, H2O and HCl.
(2) Reduction: SO2, CuO and MnO2