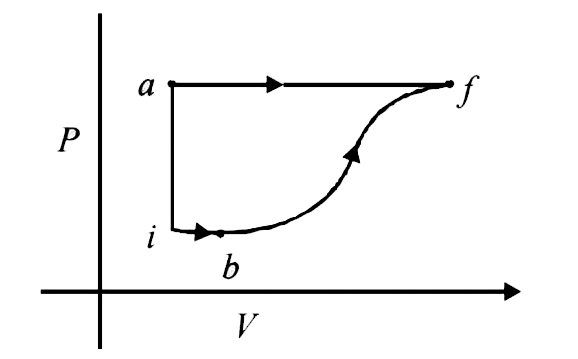
A thermodynamic system is taken from an initial state I with internal energy `U_i=-100J` to the final state f along two different paths iaf and ibf, as schematically shown in the figure. The work done by the system along the pat af, ib and bf are `W_(af)=200J, W_(ib)=50J and W_(bf)=100J` respectively. The heat supplied to the system along the path iaf, ib and bf are `Q_(iaf), Q_(ib),Q_(bf)` respectively. If the internal energy of the system in the state b is `U_b=200J and Q_(iaf)=500J`, The ratio `(Q_(bf))/(Q_(ib))` is
A. 1
B. 2
C. 3
D. 4