
(a) Geometric isomerism:
This type of isomerism is common in heteroleptic complexes. It arises due to the different possible geometric arrangements of the ligands. For example:
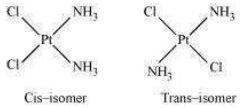
(b) Optical isomerism:
This type of isomerism arises in chiral molecules. Isomers are mirror images of each other and are non-superimposable.

(c) Linkage isomerism: This type of isomerism is found in complexes that contain
ambidentate ligands. For example:
[Co(NH3)5 (NO2)]Cl2 and [Co(NH3)5 (ONO)Cl2
Yellow form Red form (d)
Coordination isomerism:
This type of isomerism arises when the ligands are interchanged between cationic and anionic entities of differnet metal ions present in the complex.
[Co(NH3)6] [Cr(CN)6] and [Cr(NH3)6] [Co(CN)6] (e)
Ionization isomerism:
This type of isomerism arises when a counter ion replaces a ligand within the coordination
sphere. Thus, complexes that have the same composition, but furnish different ions when
dissolved in water are called ionization isomers. For e.g.,
Co(NH3)5SO4)Br and Co(NH3)5Br]SO4.
(f) Solvate isomerism:
Solvate isomers differ by whether or not the solvent molecule is directly bonded to the metal ion or merely present as a free solvent molecule in the crystal lattice.
[Cr[H2O)6]Cl3 [Cr(H2O)5Cl]Cl2 H2O [Cr(H2O)5Cl2]Cl2H2O
Violet Blue-green Dark green