Alcohols having only one hydroxyl group in their molecules are called monohydric alcohols. Monohydric alcohols are classified according to the type of hybridization of the carbon atom to which the hydroxyl group is attached.
(1) Alcohols containing Csp3 – OH bond :
In these alcohols -OH group is attached to a sp3 – hybridised carbon atom of alkyl group.
These alcohols are represented as R-OH. They are further classified as primary, secondary and tertiary alcohols in which – OH group is attached to primary, secondary and tertiary carbon atoms respectively.
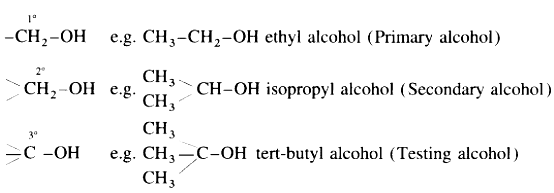
(a) Allylic alcohols : In these alcohols -OH group is attached to a sp3 -hybridised carbon atom next to the carbon-carbon double bond i.e., to allylic carbon. Allylic alcohols may be primary, secondary and tertiary alcohols.
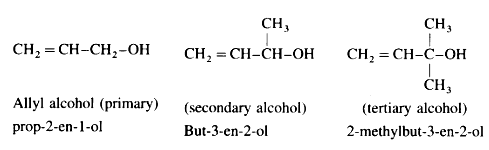
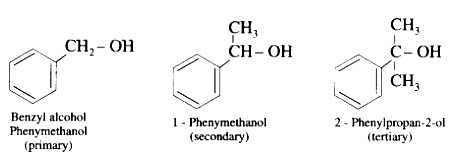
(b) Benzylic alcohols : In these alcohols -OH group is attached to a sp3 -hybridised carbon atom next to an aromatic ring. Benzylic alcohols may be primary, secondary and tertiary alcohols.
(2) Alcohols containing Csp3 – OH bond :
In these alcohols -OH group is attached to a sp2 – hybridised carbon atom,
i.e., vinylic carbon.
These alcohols are also called vinylic alcohols.
e.g., CH2 = CH – OH vinyl alcohol.