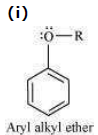
In aryl alkyl ethers, due to the +R effect of the alkoxy group, the electron density in the benzene ring increases as shown in the following resonance structure.

Thus, benzene is activated towards electrophilic substitution by the alkoxy group.
(ii) It can also be observed from the resonance structures that the electron density increases more at the ortho and para positions than at the meta position. As a result, the incoming substituents are directed to the ortho and para positions in the benzene ring.