i.

ii.

iii.

iv.

v.

The hemiacetal

is formed reversibly, while electrophilic substitution of phenols by carbonyl compound in `H^(o+)` gives irreversibly formed product (I) which futher reacts with phenol to give (C).

vi.

vii. (B) (Trialkyloxonium salts) are good alkylating agents. `SN^(2)` reaction occurs with the nucleophile `(BuOH)` displacing `Et_(2)O` (a good leaving group).

vii.

There is no change in the configuration of (C), i.e., retention of the configuration occurs since `(C---O)` bond is not broken.
ix. `BBr_(3)` form complexes with the ether

It plays a role similar to H in HI.

The products are: `MeBr + PhOH + H_(3)BO_(3)`.
x.

In (A), the axial-like encricled H atom hinder the endo approach (same side) by bulky MCPBA, so it approaches form other side, given exo-epoxide (C). The intermediate bromonium ion is also exo. It is opened by an attack with the (OH) endo, which can give only endo epoxide (B).
xi.

xii.

xiii. Commercial method for the synthesis of glycerol:

xiv.

xv.

xvi.

xvii. Dialkyl lithium cuperate is less reactive than Grignard regent, hence `(C=O)` group is not reduced by `R_(2)CuLi`.
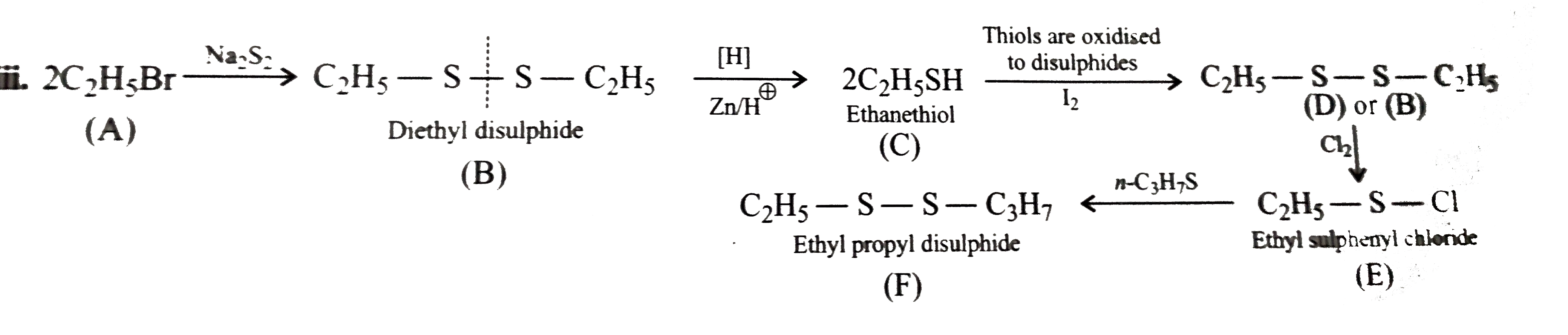
xviii.

xix.

xx.

xxi.

xxii.
