Correct Answer - C
For second ionisation potential, electron will have to be removed from valence shell of the following ions:
`C^(+)(5e^(-))=1s^(2) 2s^(2)`

`N^(+)(6e^(-))=1s^(2) 2s^(2)`

`O^(+) (7e^(-))=1s^(2) 2s^(2)`

`F^(+) (8e^(-)=1s^(2) 2s^(2)`
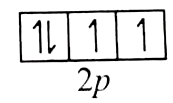
In general, ionisation energy increases from let to right in a period. However, exception occur between adjacent atoms in a period, greater amount energy is required for removal of electron from completely half-filled or completely filled orbital than the same for adjacent atom with either less than completely half -filled or less than comletely filled orbital. Therefore, ionisation potential of `O^(+)` is greater than that of `F^(+)`.
Also ionisation potential of `N^(+)` is greater than `C^(+)` but less than both `O^(+)` and `F^(+)` (periodic trend). Hence, overall order is 2nd IP: `O gt F gt N gt C`.