Correct option(a) unimolecular reaction
Explanation:
There are two different reactants (say A and B).
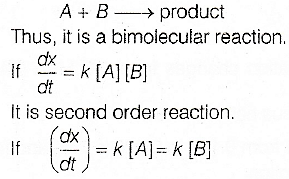
It is first order reaction.
Molecularity is independent of rate but is the sum of the reacting substances. Thus, it cannot be a unimolecular reaction.